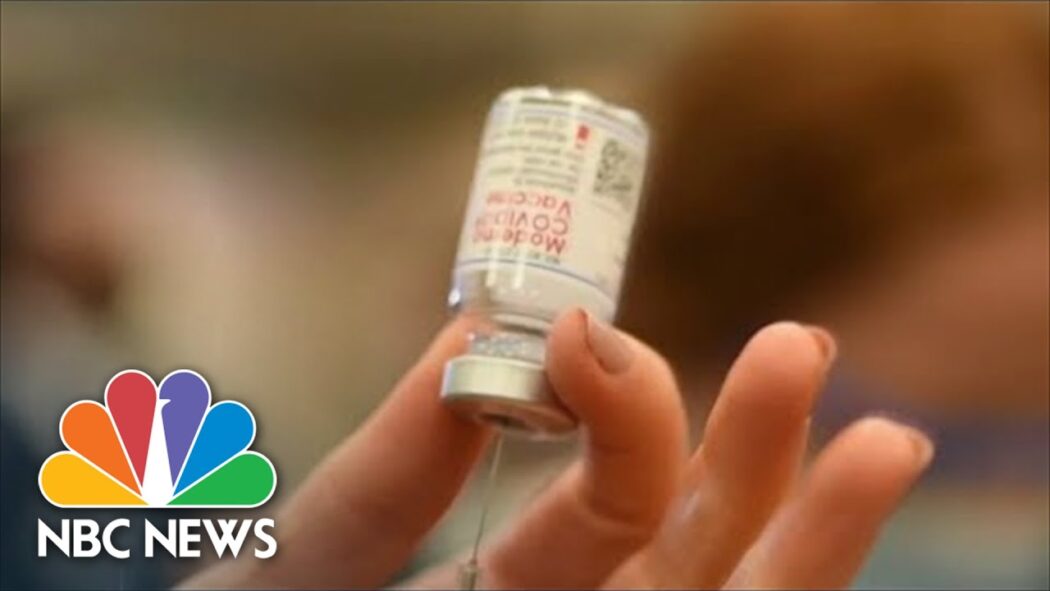
Moderna Seeks Emergency Use Authorization For Covid-19 Vaccine For Kids Under Age 6
Moderna is seeking emergency use authorization for a version of their Covid-19 vaccine for children ages six-months through five-years-old. The vaccine would be two shots taken four weeks apart, and is 25 percent of the adult dose. While less than a third of kids aged five to eleven have gotten two doses of the current vaccine available, the FDA could authorize this pediatric vaccine as early as June. » Subscribe to NBC News: http://nbcnews.to/SubscribeToNBC » Watch more NBC video: http://bit.ly/MoreNBCNews NBC News Digital is a collection of innovative and powerful news brands that deliver compelling, diverse and engaging news stories. NBC News Digital features NBCNews.com, MSNBC.com, TODAY.com, Nightly News, Meet the Press, Dateline, and the existing apps and digital extensions of these respective properties. We deliver the best in breaking news, live video coverage, original journalism and segments from your favorite NBC News Shows. Connect with NBC News Online! NBC News App: https://smart.link/5d0cd9df61b80 Breaking News Alerts: https://link.nbcnews.com/join/5cj/breaking-news-signup?cid=sm_npd_nn_yt_bn-clip_190621 Visit NBCNews.Com: http://nbcnews.to/ReadNBC Find NBC News on Facebook: http://nbcnews.to/LikeNBC Follow NBC News on Twitter: http://nbcnews.to/FollowNBC Moderna Seeks Emergency Use Authorization For Covid-19 Vaccine For Kids Under Age 6
Try Adsterra Earnings, it’s 100% Authentic to make money more and more.

*NBC News*
Moderna Seeks Emergency Use Authorization For Covid-19 Vaccine For Kids Under Age 6
Full/More Story on Source:
Published By

Latest entries
 allPost2025.01.27Twin brothers reunited in Gaza City after being separated for over a year
allPost2025.01.27Twin brothers reunited in Gaza City after being separated for over a year allPost2025.01.27Mark 80 years since the liberation of Auschwitz
allPost2025.01.27Mark 80 years since the liberation of Auschwitz allPost2025.01.27Loto Club Страна вашинский вероятность выиграть до пятидесяти миллиона KZT!
allPost2025.01.27Loto Club Страна вашинский вероятность выиграть до пятидесяти миллиона KZT! allPost2025.01.27The Best Way To slots and blackjack
allPost2025.01.27The Best Way To slots and blackjack